Lithium batteries have become the lifeblood of modern technology. From the smartphone in your pocket to the electric vehicle (EV) in your driveway, these energy storage devices are ubiquitous. However, their rise to dominance has been accompanied by a shadow: the risk of fire and explosion. While the vast majority of lithium batteries operate safely, specific types, conditions, and chemistries pose significant dangers.
Understanding which lithium batteries are dangerous requires looking beyond just the brand name. It involves examining the chemical composition, the quality of manufacturing, the physical design, and the conditions under which they are used. This article delves deep into the specific categories and scenarios where lithium batteries transition from useful tools to potential hazards.
The Chemistry of Risk: Not All Lithium is Created Equal
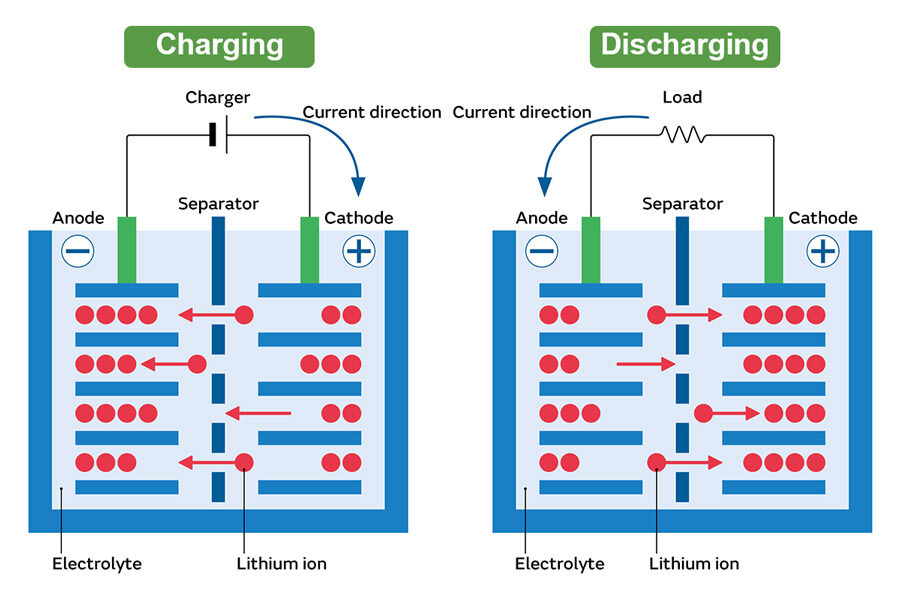
To understand the danger, one must first understand the chemistry. Lithium-ion (Li-ion) batteries function by moving lithium ions between the cathode and anode during charge and discharge cycles. The liquid electrolyte that facilitates this movement is typically organic and highly flammable. When a battery fails, it can enter a state called thermal runaway, a rapid, self-sustaining increase in temperature that often leads to fire or explosion.
However, different chemical compositions have different stability profiles:
1. Lithium Cobalt Oxide (LCO)
Found commonly in older laptops, cameras, and some smartphones, LCO batteries offer high energy density—meaning they pack a lot of power into a small space. However, they are chemically less stable than newer alternatives. They have a relatively low thermal runaway temperature (around 150°C / 302°F). If overcharged or physically damaged, LCO batteries are among the most likely to catch fire aggressively.
2. Lithium Nickel Manganese Cobalt Oxide (NMC)
NMC batteries are the current standard for many electric vehicles and power tools. They balance power, energy density, and safety better than LCO. However, they are still susceptible to thermal runaway if the separator between the anode and cathode is breached. Because EVs use massive packs containing thousands of these cells, a failure in a single cell can propagate to others, creating a massive, hard-to-extinguish fire.
3. Lithium Iron Phosphate (LFP)
The Safer Alternative: It is important to note that LFP batteries are widely considered the safest mainstream lithium chemistry. They have a much higher thermal runaway threshold (around 270°C / 518°F) and are very difficult to ignite even when punctured. While no battery is 100% risk-free, LFP represents the lower end of the danger spectrum.
The Danger of Form Factor: Pouch vs. Cylindrical
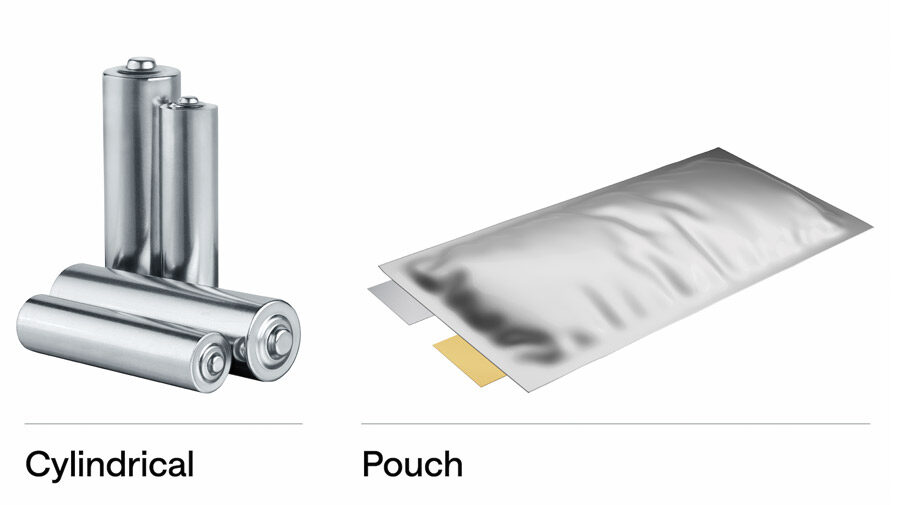
The physical shape of the battery also dictates its safety profile and susceptibility to damage.
-
Pouch Cells: These are the flat, foil-enclosed batteries found in smartphones and slim laptops. Because they lack a rigid metal casing, they are highly vulnerable to physical trauma. Puncturing a pouch cell almost immediately causes a short circuit and fire. Furthermore, as they age or fail, they tend to "swell" due to gas buildup (a phenomenon known as "pillowing"), which is a clear precursor to potential rupture and ignition.
-
Cylindrical Cells (e.g., 18650, 21700): These look like traditional AA batteries but are slightly larger. They have a steel casing that provides excellent mechanical protection. However, if the internal pressure vents fail during a malfunction, the steel casing can essentially turn the battery into a pipe bomb.
The "Grey Market" and Counterfeit Batteries
Perhaps the most dangerous lithium batteries are not those with a specific chemistry, but those with a specific origin: Cheap, unbranded, or counterfeit replacements.
Major manufacturers (like Panasonic, LG, Samsung, CATL) implement rigorous quality control, including safety vents, thermal fuses, and high-quality separators. Grey market batteries often skip these expensive safety features to cut costs.
-
Missing Battery Management Systems (BMS): A BMS is the brain of the battery pack. It prevents overcharging, over-discharging, and overheating. Cheap replacement batteries for e-bikes and scooters often have rudimentary or non-existent BMS protection.
-
Impure Materials: Contaminants in the electrolyte or electrode materials can cause internal short circuits (dendrite growth) over time, leading to spontaneous fires even when the device is turned off.
Key Insight: A significant percentage of e-bike and e-scooter fires in urban apartments are traced back to aftermarket, low-quality battery packs or chargers that were not designed for the specific battery.
Specific Scenarios That Make Batteries Dangerous
Even a high-quality battery can become a lethal hazard under specific conditions.
1. Physical Damage
If a lithium battery is crushed, punctured, or bent, the separator between the anode and cathode breaks. This causes a direct internal short circuit. The massive flow of energy creates instant heat, igniting the electrolyte. This is why you must never use a phone that is bent or an EV that has suffered severe undercarriage damage without professional inspection.
2. Improper Charging (The "Vampire" Draw)
Using a charger with a higher voltage than the battery is rated for, or a charger that does not cut off power when the battery is full, forces energy into a cell that cannot hold it. This creates lithium plating and excess heat. Leaving cheap e-scooters charging overnight in hallways is a leading cause of residential fires.
- Heat: Storing batteries in a hot car degrades the separator and lowers the threshold for thermal runaway.
Note: As indicated above, storing a battery in high temperatures (like a hot car) significantly lowers its safety margin. A battery that is stable at room temperature may spontaneously enter thermal runaway at much lower internal temperatures if the separator has been degraded by heat.
Comparative Analysis of Lithium Battery Risks
The following table categorizes common lithium battery types and their associated risk levels to help identify which are most dangerous in daily life.
|
Battery Chemistry / Type
|
Common Applications
|
Risk Level
|
Primary Danger Factors
|
Thermal Runaway Threshold
|
|
Lithium Cobalt Oxide (LCO)
|
Older laptops, cameras, some phones
|
High
|
Low thermal stability; highly reactive if damaged or overcharged.
|
~150°C (302°F)
|
|
Pouch Cells (General)
|
Smartphones, tablets, ultra-thin laptops
|
Medium-High
|
Vulnerable to puncture; prone to swelling (gas buildup); lack of rigid protection.
|
Varies by chemistry
|
|
Unbranded / Grey Market
|
E-bikes, replacement scooter batteries, cheap toys
|
Very High
|
Lack of quality control; missing BMS; impure materials; poor welding.
|
Unpredictable
|
|
NMC (Nickel Manganese Cobalt)
|
Power tools, most Electric Vehicles, e-bikes
|
Medium
|
High energy density means intense fires if they occur; requires sophisticated BMS.
|
~210°C (410°F)
|
|
Loose 18650 Cells (Vaping)
|
Vaping devices, high-end flashlights
|
High
|
Often carried in pockets with keys/coins causing shorts; plastic wraps peel easily.
|
~210°C (410°F)
|
|
Lithium Iron Phosphate (LFP)
|
Newer EVs (Standard Range), solar storage, golf carts
|
Low
|
Highly stable chemical structure; difficult to ignite even when punctured.
|
~270°C (518°F)
|
The Danger of "Button" Batteries
While we often focus on large fires, small lithium coin cells (often found in watches, car keys, and remotes) pose a different, biological danger. If swallowed by a child or pet, the electrical current reacts with bodily fluids (saliva/mucus) to create hydroxide, effectively burning through the esophagus or stomach lining within hours. While they do not typically explode, they are "dangerous" in a way that is lethal to living tissue.







