Contents:
A wet battery, or liquid-electrolyte battery, is one of the earliest and most important forms of chemical power sources ever developed. Long before modern portable electronics made dry cells and lithium-ion batteries famous, wet batteries were providing reliable energy for telegraphs, early laboratories, and later for cars, backup power systems, and industrial applications. Even today, when you turn the key in a conventional car and the engine starts, you are almost certainly relying on a wet battery: the lead–acid starter battery under the hood.
Definition and Basic Structure
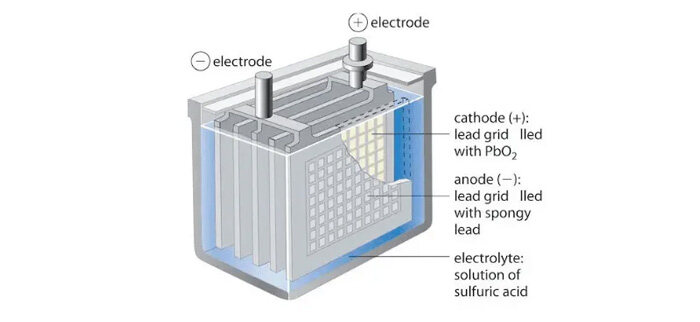
A wet battery (also called a liquid-electrolyte battery or, more generally, a wet cell) is an electrochemical cell in which the electrolyte is a liquid solution. This liquid contains ions that can move freely between the electrodes, enabling chemical reactions that produce electrical energy.
A typical wet battery has four main components:
-
Positive electrode (cathode during discharge)
-
Negative electrode (anode during discharge)
-
Electrolyte (liquid)
-
Container or casing
Wet Battery vs. Dry Battery
A dry battery (dry cell), such as the common AA or AAA alkaline cell, uses a paste or gel-like electrolyte, rather than a free-flowing liquid. Key differences:
| Feature |
Wet Battery (Liquid Electrolyte) |
Dry Battery (Paste / Gel Electrolyte) |
| Electrolyte state |
Free-flowing liquid |
Thick paste or gel |
| Orientation sensitivity |
Often must be kept upright to avoid leakage |
Can usually operate in any orientation |
| Maintenance |
Often requires checking fluid levels, cleaning terminals |
Typically maintenance-free |
| Common uses |
Car batteries, backup power, industrial systems |
Flashlights, remote controls, portable gadgets |
| Rechargeability |
Many types are rechargeable (e.g., lead–acid) |
Some are rechargeable (NiMH, Li-ion), many are disposable |
In short, wet batteries prioritize current capacity and robustness over portability and convenience, while dry batteries focus on safe, compact, orientation-independent operation.
How a Wet Battery Works
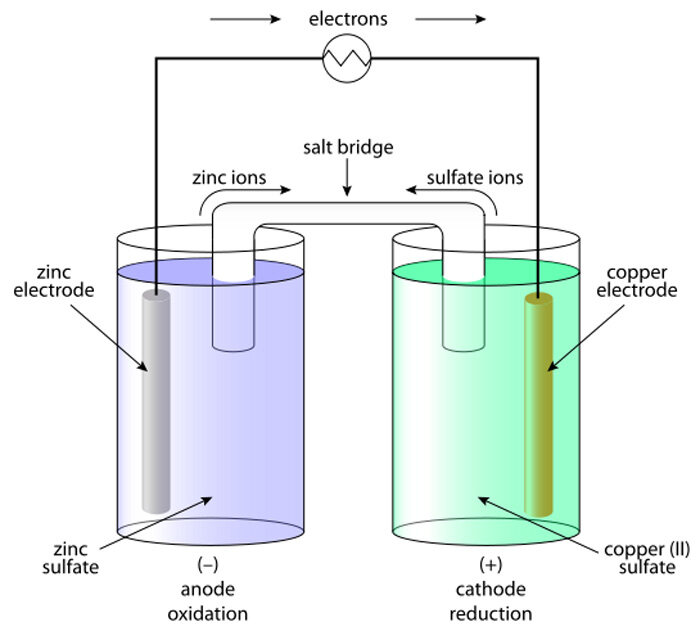
The operation of a wet battery is governed by electrochemical reactions occurring at the electrodes in contact with the liquid electrolyte.
Basic Electrochemical Concept
Inside the battery:
-
At the negative electrode, a chemical reaction releases electrons into the external circuit and produces positive ions into the electrolyte (oxidation).
-
At the positive electrode, another reaction consumes electrons from the external circuit and positive ions from the electrolyte (reduction).
Because electrons cannot pass through the electrolyte, they travel through the external circuit, powering any device connected between the terminals. At the same time, ions move through the electrolyte to maintain charge balance.
Electron Flow and Current
When the battery is connected to a load (e.g., a motor, a light bulb):
-
Electrons flow out of the negative terminal, through the external wires.
-
The electrons pass through the load, doing electrical work (e.g., lighting a bulb).
-
They then enter the positive terminal and participate in reduction reactions.
The direction of conventional current is opposite to electron flow: it is considered to flow from the positive terminal to the negative terminal in the external circuit.
Factors Affecting Voltage and Capacity
Several interrelated factors determine the voltage (potential difference) and capacity (how much charge or energy the battery can deliver) of a wet battery:
-
Electrode materials
-
Different combinations of metals and compounds have different inherent electrode potentials.
-
For example, the lead–acid system has a nominal cell voltage of about 2.0 V per cell, while other chemistries (like nickel–cadmium) have different per-cell voltages.
-
Electrolyte composition and concentration
-
The type of ions and their concentration in the solution strongly influence the equilibrium potentials at each electrode.
-
According to basic electrochemistry (e.g., the Nernst equation), the cell voltage depends on the concentrations (or activities) of reactants and products.
-
Temperature
-
Temperature affects reaction rates and ion mobility.
-
Higher temperatures generally increase capacity and reduce internal resistance, but accelerate degradation and can pose safety risks.
-
Internal resistance and design
-
The geometry of the electrodes, separator type, and distance between plates contribute to internal resistance.
-
Lower internal resistance allows higher current output but may increase heat generation.
-
State of charge and aging
-
As a battery discharges, reactant concentrations change and reaction products accumulate, slightly lowering the voltage.
-
Over time, electrode surfaces may degrade or become contaminated, reducing capacity.
Conceptual Line Chart: Effect of Electrolyte Concentration
Below is a conceptual example of how electrolyte concentration can influence both cell voltage and capacity in a wet battery. This is not data from a specific battery model, but an illustrative trend:
In this illustration:
-
As the electrolyte concentration increases from very low values, the voltage gradually rises and then levels off. At very low concentration, there are not enough ions to support the ideal electrochemical potential.
-
The capacity initially increases because better ionic conductivity reduces internal losses and allows more of the active material to participate.
-
Beyond an optimal concentration, capacity may start to fall slightly due to increased viscosity, diffusion limitations, or accelerated side reactions that damage the electrodes.
Types and Common Examples
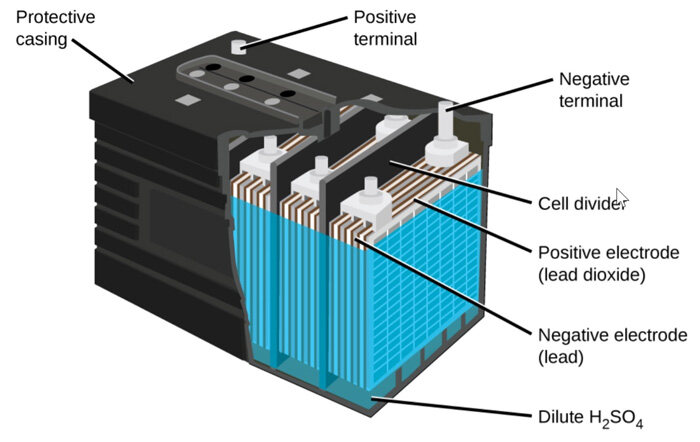
Wet batteries come in several forms, but some classic and widely used types stand out.
Lead–Acid Batteries
The lead–acid battery is the most common wet battery today, especially as a starter battery in automobiles.
-
Positive electrode: lead dioxide (PbO₂)
-
Negative electrode: porous lead (Pb)
-
Electrolyte: sulfuric acid solution (H₂SO₄ in water)
-
Nominal cell voltage: ~2.0 V per cell
-
Typical car battery: 12 V system using six cells in series
Applications include:
-
Car starting, lighting, and ignition (SLI batteries)
-
Backup power in uninterruptible power supplies (UPS)
-
Energy storage in some off-grid or backup power systems
Classic Wet Cells: Daniell Cell and Others
Historically, the Daniell cell was an early wet cell used in telegraph systems and laboratories.
-
Positive half-cell: copper electrode in copper sulfate solution
-
Negative half-cell: zinc electrode in zinc sulfate solution
-
The two solutions are separated, often by a porous barrier or a salt bridge.
This cell was more stable and provided a more constant voltage than very early systems like the simple voltaic pile, making it extremely important in the 19th century.
Everyday Applications
Even if we don’t always see them, wet batteries appear in many daily contexts:
-
Automobiles and motorcycles
-
Boats and marine applications
-
Backup lighting and emergency power systems
-
Industrial forklifts and traction batteries (in flooded lead–acid variants)
-
Telecom towers and data centers, where large banks of wet batteries ensure continuous power
Comparison Table of Typical Wet Battery Examples
| Type |
Electrodes |
Electrolyte |
Nominal Cell Voltage |
Typical Use Case |
| Lead–acid (SLI) |
Pb / PbO₂ |
H₂SO₄ solution |
~2.0 V |
Car starter batteries |
| Flooded traction |
Pb / PbO₂ (thicker plates) |
H₂SO₄ solution |
~2.0 V |
Forklifts, industrial vehicles |
| Daniell cell |
Zn / Cu |
ZnSO₄ and CuSO₄ solutions |
~1.1 V |
Historical labs, telegraph systems |
| Other wet cells |
Various metal couples (e.g., Zn/Fe) |
Aqueous salt or acid solutions |
Varies |
Early experiments, specialty applications |
Advantages of Wet Batteries
Wet batteries have several important advantages that have kept them relevant for more than a century:
-
Relatively low manufacturing cost
-
The materials (e.g., lead and sulfuric acid) are abundant and relatively inexpensive.
-
Established manufacturing processes and economies of scale further reduce cost.
-
High surge current capability
-
Wet batteries, especially flooded lead–acid designs, can deliver very high currents for short periods.
-
This makes them ideal for engine starting, where a powerful surge is needed to crank the motor.
-
Rechargeable and reusable
-
Many wet batteries (like lead–acid) are rechargeable, allowing hundreds or even thousands of charge–discharge cycles if properly maintained.
-
This reduces long-term cost and environmental impact compared to disposable cells.
-
Rugged and well-understood technology
-
The chemistry of lead–acid and similar systems is mature and well-characterized.
-
Properly designed wet batteries can be robust in harsh environments, with wide operating temperature ranges.
Disadvantages and Safety Issues
Despite their strengths, wet batteries have drawbacks and require care in handling and maintenance.
Leakage and Corrosive Electrolyte
The liquid electrolyte, often an acid:
-
Can leak if the battery is tipped, damaged, or overfilled.
-
Is corrosive to skin, eyes, and many materials (e.g., metals, clothing, paint).
-
Can cause corrosion on terminals and surrounding metal parts, especially in vehicles.
Regular inspection and cleaning of the terminals can help reduce corrosion and ensure a reliable connection.
Size, Weight, and Portability
Wet batteries tend to be:
-
Heavy, due to thick plates and large amounts of liquid electrolyte.
-
Bulky, making them less suitable for handheld devices.
This makes them excellent for stationary or vehicle-mounted use, but not ideal where space and weight are at a premium (e.g., smartphones, laptops).
Maintenance Requirements
Many traditional wet batteries, especially flooded lead–acid types, require regular maintenance:
-
Checking electrolyte levels
-
Water can be lost due to electrolysis and evaporation, especially under high charging voltages.
-
Distilled water may need to be added periodically to keep plates submerged.
-
Inspecting and cleaning terminals
-
Monitoring charge condition
Sealed variants (like valve-regulated lead–acid, VRLA) reduce these requirements but are often still based on wet-system chemistry.
Safety Precautions
Because of their energy content and chemicals, wet batteries must be handled with care:
-
Prevent short circuits
-
Tools or metal objects bridging the terminals can cause dangerous high currents, sparks, and heating.
-
Always avoid placing metal objects on top of batteries.
-
Avoid tipping and rough handling
-
Ventilation
-
During charging, some wet batteries release gases, including hydrogen.
-
Adequate ventilation is essential to avoid explosive gas buildup.
-
Personal protective equipment (PPE)
-
When working with open wet batteries, use gloves and eye protection.
-
Be prepared to neutralize small acid spills and rinse skin or eyes with plenty of water if contact occurs.
FAQs
How does the internal resistance of a wet battery compare with other battery types? +
Wet batteries typically exhibit lower internal resistance than many dry cells or sealed batteries. This is due to the high ionic mobility in their liquid electrolyte and the larger electrode surface area. Lower internal resistance allows wet batteries to deliver high surge currents, which is why they are commonly used in engine starting and other high-demand applications.
What environmental considerations are associated with wet batteries? +
Wet batteries—especially lead–acid types—require responsible handling and recycling. The lead plates and sulfuric acid electrolyte are classified as hazardous materials, but the industry has one of the highest recycling rates worldwide (often above 95%). Proper recycling prevents soil and water contamination and allows recovery of valuable materials like lead and polypropylene.
How do wet batteries handle overcharging, and what risks are involved? +
Overcharging a wet battery can cause electrolysis of the water in the electrolyte, producing hydrogen and oxygen gas. This leads to water loss (requiring refilling), increased internal pressure, higher risk of explosion if ventilation is poor, and accelerated corrosion of plates and grids. Proper chargers with voltage regulation and temperature compensation help prevent overcharge damage.
Can wet batteries operate efficiently in extreme temperatures? +
Wet batteries generally perform well across a broad temperature range, but extremes affect performance. Low temperatures reduce ion mobility, decreasing available cranking power. High temperatures improve capacity short term but accelerate electrode corrosion and electrolyte evaporation, shortening battery life. Thermal management or insulated enclosures are often used in industrial or automotive applications.
What are the differences between flooded wet batteries and absorbed glass mat (AGM) systems? +
Although both are technically lead–acid systems, their structure differs significantly. Flooded wet batteries use freely circulating liquid electrolyte and require periodic maintenance. AGM batteries immobilize electrolyte in fiberglass mats, offering lower self-discharge, higher vibration resistance, faster charge acceptance, and minimal maintenance. They are often chosen for start-stop vehicles, marine systems, and backup power applications.









