In the race to develop next-generation batteries, Chinese scientists have taken several major steps toward solving one of the toughest challenges in the field: how to make solid-state lithium-metal batteries both powerful and practical.
These new advances, achieved by multiple research teams across China, could finally remove a critical roadblock that has long prevented solid-state batteries from entering large-scale commercial use. The results hint at a future where electric vehicles could travel over 1,000 kilometers (620 miles) on a single charge — safely, efficiently, and without relying on flammable liquid electrolytes.
1. The “Special Glue”: Iodine Ions That Fill the Cracks
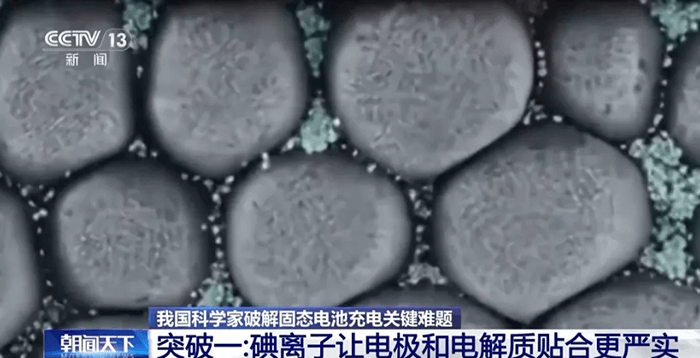
A team from the Institute of Physics at the Chinese Academy of Sciences, working with several partner universities, has developed what they call a “special glue” for solid-state batteries — a self-healing interface material based on iodine ions.
Here’s how it works:
When the battery is operating, iodine ions move toward the interface between the electrode and the solid electrolyte under the influence of the electric field. Once there, they act like tiny repair workers — or liquid sealant — automatically finding and filling any small cracks or voids.
The iodine ions also help attract lithium ions, ensuring a smooth and stable pathway for their movement. Over time, this dynamic self-adjustment allows the two solid layers to “fit together” more tightly, eliminating performance losses caused by poor contact.
Researchers describe this as “an interface that can heal itself in real time” — a crucial feature for any battery expected to survive thousands of charge and discharge cycles. It’s an elegant chemical solution to a mechanical problem, and early tests show substantial improvements in both energy density and cycling stability.
2. The “Flexible Transformation”: A Polymer Skeleton That Bends Without Breaking
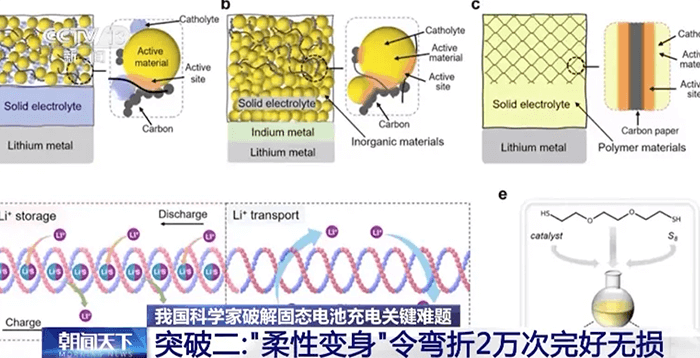
At the Institute of Metal Research under the Chinese Academy of Sciences, another team tackled the problem from a mechanical perspective. Their solution gives the brittle ceramic electrolyte a flexible polymer skeleton, turning it into what they call a “stretchable solid”.
Imagine upgrading a fragile sheet of ceramic into something like a reinforced plastic wrap — thin, strong, and flexible. This hybrid material can be twisted, folded, or even bent 20,000 times without cracking. Tests show that even when the cell is twisted into a corkscrew shape, it remains fully functional.
The key lies in the chemical design of the polymer skeleton. Within it, the researchers embedded molecular “micro-components” that perform two additional roles:
-
Some components help lithium ions move faster, increasing ion conductivity.
-
Others can trap and store more lithium ions, effectively increasing the battery’s charge capacity.
Combined, these effects lead to a remarkable 86% improvement in energy storage capacity compared with earlier solid-state designs. The new structure allows the battery to maintain performance under mechanical stress — an essential step toward flexible or foldable electronics, as well as safer electric vehicles.
3. “Fluorine Reinforcement”: Building a Protective Shell to Resist High Voltage
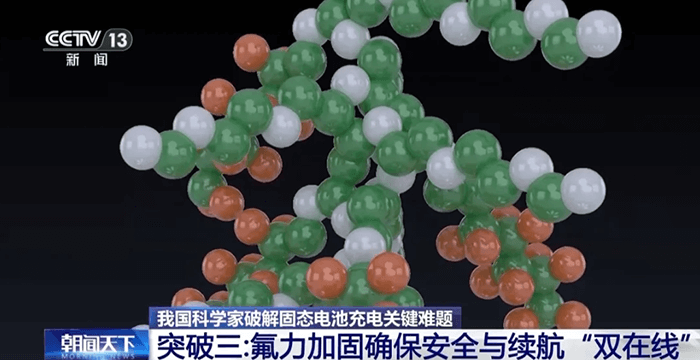
The third breakthrough comes from a research group at Tsinghua University, which has been exploring how to make solid electrolytes more resistant to high voltages — a major cause of degradation and short circuits.
Their solution is a fluorine-based polymer modification, using fluoropolyether materials. Fluorine atoms are famous for their strength: the carbon-fluorine bond is one of the toughest in chemistry. By incorporating these fluorinated polymers into the electrolyte, the researchers were able to form a protective fluoride shell on the surface of the electrode.
This shell acts as a high-voltage armor, preventing electrical breakdowns and chemical side reactions that can destroy the electrolyte during fast charging or at full charge.
Safety tests are equally impressive. The modified solid-state cells reportedly passed needle penetration tests and 120°C high-temperature oven tests without explosion or fire — conditions that would easily ignite conventional liquid lithium batteries. The combination of safety and performance makes the approach one of the most promising among current solid-state technologies.








